Cancer is now seen as a disease which arises due to a failure of body immunity to detect the abnormal cells or the cancer cells. With the advancement in the medical science, we are now entering a new era where cancer will be treated by inducing our own immunity to kill these cancer cells. The current most effective implementation of this immunotherapy is in form of CART (Chimeric Antigen Receptor T Cell) therapy.
CART & Mechanism of Action
Till recently one of the best ways of immunotherapy was the hematopoietic stem cell transplantation which gives the body a new immunity and thus produces action against the leukemic cells. This is termed as graft Vs leukemia effect or simply GVL effect. However, this is associated with immune action against the normal body cells which is GVHD (Graft Vs Host Disease). CART therapy involves the use of patient own T cell to produce action against some specific antigens thus sparing normal body cells.
The patient’s own body T cells are isolated using apheresis from peripheral blood and they are genetically engineered by adoptive gene transfer techniques to express specific receptors which can identify the abnormal cells or the leukemic cells and produce an immune action against them. They can further be divided into many types or generation depending on the presence of co-stimulatory molecules and cytokine genes which determine their effectiveness.
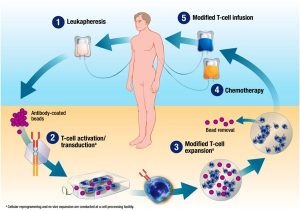
Therefore CART therapy should be termed as “personalized chemotherapy” as for every patient, separate CART will be produced.
Tisagenlecleucel- CART against CD19
CD19 is the molecule that is expressed on all the leukemic cells of B lymphoblastic leukemia. Producing CART therapy against this molecule is expected to produce an action against the leukemic cells of B lymphoblastic leukemia. The clinical trials conducted on relapsed refractory cases of B lymphoblastic leukemia showing ~ 90% remission rates. Owing to such impressive results FDA has now approved TISAGENLECLEUCEL use in cases of relapsed and refractory B Lymphoblastic leukemia.
With the trials on the way for other hematological malignancies using CART therapy, we can surely say that the days of conventional and toxic chemotherapy are few.
Last modified: 21/07/2017