Diffuse gliomas are the most common primary brain tumor in adults. While several important genetic alterations in gliomas have been known for some time, deeper genetic and epigenetic studies of large numbers of glioma samples with the help of new tools, has lead to a number of novel discoveries in the recent years. One of the most exciting and clinically relevant observations was the discovery that a high percentage of lower grade diffuse gliomas i.e. grade II and III astrocytomas and oligodendrogliomas and secondary glioblastoma tumors (and a lower percentage of higher grade gliomas) harbor mutations in the genes isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2). However, these mutations are rare in primary glioblastoma.
Various studies indicate that these mutations play a causal role in gliomagenesis, have a major impact on tumor biology, and have clinical and prognostic importance.
The IDH1 and IDH2 genes are located on chromosome 2q33.3 and 15q26.1, respectively. The IDH enzymes are involved in a number of metabolic processes including mitochondrial oxidative phosphorylation, glutamine metabolism, lipogenesis, glucose sensing, and regulation of cellular redox status.
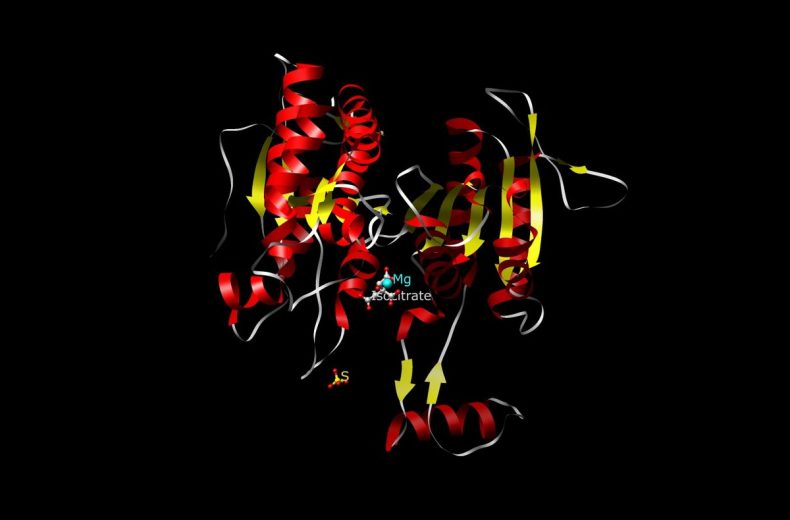
 IDH1 and IDH2 are highly similar proteins, located in the cytosol and mitochondria, respectively, both catalyzing the reversible oxidative decarboxylation of isocitrate to alpha-ketoglutarate (αKG) while reducing NADP+ to NADPH. Most mutations (>90%) identified to date have been a single amino acid missense mutation in IDH1 at arginine 132 (R132) or the analogous residue in IDH2 (R172). The mutant forms of IDH1 and IDH2 lead to the production of the oncometabolite 2-hydroxyglutarate, which inhibits the function of numerous α-ketoglutarate–dependent enzymes. Inhibition of the family of histone demethylases and the TET family of 5-methylcytosine hydroxylases profoundly affects the epigenetic status of mutated cells and leads directly to a hypermethylator phenotype that has been referred to as the CpG island methylator phenotype (G-CIMP).
IDH1 and IDH2 are highly similar proteins, located in the cytosol and mitochondria, respectively, both catalyzing the reversible oxidative decarboxylation of isocitrate to alpha-ketoglutarate (αKG) while reducing NADP+ to NADPH. Most mutations (>90%) identified to date have been a single amino acid missense mutation in IDH1 at arginine 132 (R132) or the analogous residue in IDH2 (R172). The mutant forms of IDH1 and IDH2 lead to the production of the oncometabolite 2-hydroxyglutarate, which inhibits the function of numerous α-ketoglutarate–dependent enzymes. Inhibition of the family of histone demethylases and the TET family of 5-methylcytosine hydroxylases profoundly affects the epigenetic status of mutated cells and leads directly to a hypermethylator phenotype that has been referred to as the CpG island methylator phenotype (G-CIMP).
Patients with gliomas with mutated IDH are several years younger & have significantly longer overall survival than patients with wild-type IDH, independent of age & grade. Also, IDH mutations are rare in gliomas in young children, although they are common in adolescents.
The presence of the IDH mutation is a Pathognomonic biomarker that indicates a glioma entity in the setting of brain tumors.
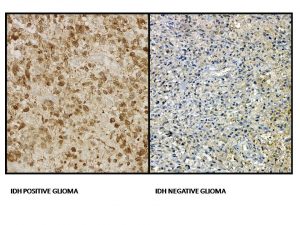
Because of the consistent protein alteration, a monoclonal antibody (IDHR132H) has been developed to the mutant protein, allowing its use for detection of this mutation in gliomas by immunohistochemistry (IHC) from paraffin-embedded sections. In mutant tumors, all neoplastic cells exhibit some degree of cytoplasmic (stronger) & nuclear (weaker) labeling. The ability of the antibody to detect a small number of cells as mutant makes this method more sensitive than sequencing for identifying the R132H mutation. Also, it is time and cost-effective as compared to DNA sequencing. However, mutations in IDH2 and other mutation is IDH1 will not be detected using IHC with this antibody & can only be identified by DNA sequencing. If IHC is negative or inconclusive in lower grade gliomas or suspected secondary glioblastoma, direct sequencing of the IDH1 and IDH2 genes by PCR or NGS based methods should be carried out to screen for non -IDH1-R132H mutations. In addition to detection of IDH mutation in tissue, the increased levels of 2-hydroxyglutarate in IDH mutated tumors have led several researchers to develop non-invasive imaging methods for detection of IDH-mutated gliomas, since 2-hydroxyglutarate has a distinctive magnetic resonance spectrum that is detectable by in vivo magnetic resonance spectroscopy (MRS). MRS may, therefore, be used for distinguishing gliosis from low-grade gliomas, potentially avoiding biopsy in ambiguous cases.
IDH mutation has been seen to be of predictive value for response to chemotherapy in anaplastic gliomas. Extended follow-up of a large randomised controlled trial that compared procarbazine/lomustine/vincristine (PCV) chemotherapy in combination with radiotherapy to radiotherapy alone demonstrated a survival advantage of the combination in IDH-mutant anaplastic gliomas. Median overall survival for patients with IDH-mutant tumors was 5.7 years for patients treated with radiotherapy alone versus 9.4 years for patients who received radiotherapy plus PCV. Conversely, the addition of PCV chemotherapy to radiotherapy did not prolong median survival in patients with IDH Wild-type tumors, (1.3 vs 1.8 years).
Additionally, IDH mutation serves as a potential therapeutic target as inhibitors of mutant IDH have entered clinical trials for patients with IDH1 mutations. AG-120 and AG-221 are first-in-class, oral, selective inhibitors of the IDH1 and IDH2 mutant enzymes, respectively. Vaccination against IDH1 mutant specific peptides is currently being investigated in IDH1-mutant gliomas in phase 1 trials.
Thus, IDH mutation testing is a valuable diagnostic, prognostic, predictive & therapeutic biomarker for the management of glial tumors.
Disclaimer: The picture(s) used in this blog is for education purpose only with no commercial usage.
Last modified: 23/08/2017