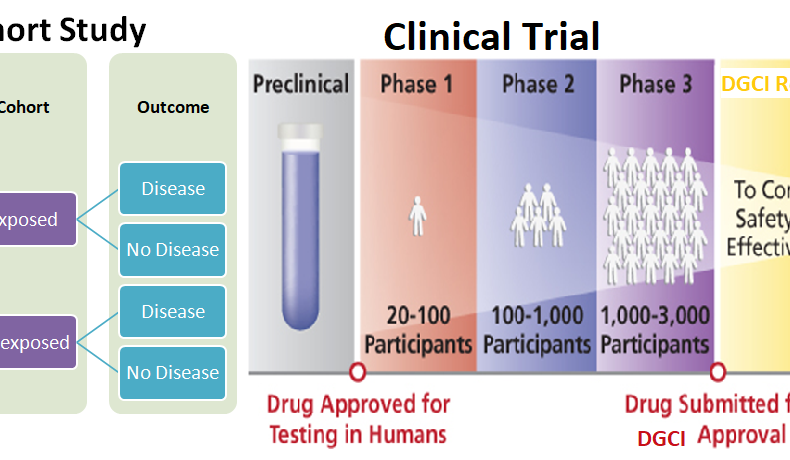
Both are prospective study designs however a cohort study is much like a clinical trial except that the intervention. In clinical trials, intervention is investigator controlled, whereas it is a naturally occurring phenomenon in a cohort study.
In a cohort study, subjects with specific characteristics and assigned exposure are being followed till getting a defined outcome. It is not necessary that the follow-up chronology is in the future, it could be the past also. Sokal et al. (1995) conducted a study in 1992 on basis of records between 1977 and 1991 and interview traceable women in future. It is not a retrospective study because investigation direction is from antecedent to outcome. Historical cohort or retrospective cohorts are some of the examples of subjects with exposure in past, followed for development of outcome.
Medical experiments on the human being are known as clinical trials. It is mostly conducted to investigate new modes of therapy. Research on diagnostic procedures, behaviour and counselling also fall into a clinical trial. All trials are prospective studies where exposure is intervention and efficacy & safety are the outcomes. A follow-up is an inbuilt feature of a clinical trial. All clinical trials involve cautious consideration of issues such as selection of subjects and controls, sample size calculation, randomization & matching and blinding, masking and concealment of allocation. There are various strengths of clinical trials however the biggest problem is Unknown confounders. Randomization with large sample size may reduce confounding effect. Also, many clinical trials are multicenter studies faces challenges like between-centre differences in standard protocols, recruitment, patient selection, culture, and socioeconomic etc. Nowadays there is a trend among researchers conducting a single-centric study to overcome such problem and produce more reliable and validated results.
There are five phases of clinical trials i.e. pre-clinical study, phase1, phase2, phase3 and phase4. It starts with pre-clinical studies on animals followed by volunteers from healthy subjects and patients. Post-marketing surveillance is to monitor the safety of a pharmaceutical drug or medical device after it has been released on the market.
In summary, cohort studies and clinical trials offer the best probability that study results reproduce the truth. However, the clinician needs to be aware of the many strengths and limitations of these study designs and approach the results of any trial with healthy scepticism.
Reference:
Sokal DC, Zipper J, Guzman-Serani R, Aldrich TE. Cancer risk among women sterilized with transcervical quinacrine hydrochloride pellets, 1977 to 1991. Fertl Steril 1995; 64:325-34
Last modified: 21/11/2019